
In order to simplify this equation, order of operations dictates that the addition of the solute and solvent volumes occurs first. Therefore, the given quantities are both expressed in the appropriate unit and can be directly incorporated into the indicated equation, as shown below. As stated above, all of the volumes that are incorporated into the equations that are shown above must be reported in milliliters. Before this equation can be applied, the validity of the units that are associated with the given numerical values must be confirmed. Alternatively, because a solution can only contain one solvent, after identifying helium, He, as the solvent in this solution, molecular chlorine, Cl 2, can be classified as the solute "by default."īecause the amount of helium, He, is specified in the given problem, the second equation that is presented above should be applied to determine the volume percent of this solution. The volume of a regular object can be calculated by multiplying its length by its width and its height. Helium, He, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 519.2 milliliters, and molecular chlorine, Cl 2, is the solute, as it is present in a lesser quantity, 168.4 milliliters. Volume is the amount of space occupied by a sample of matter.
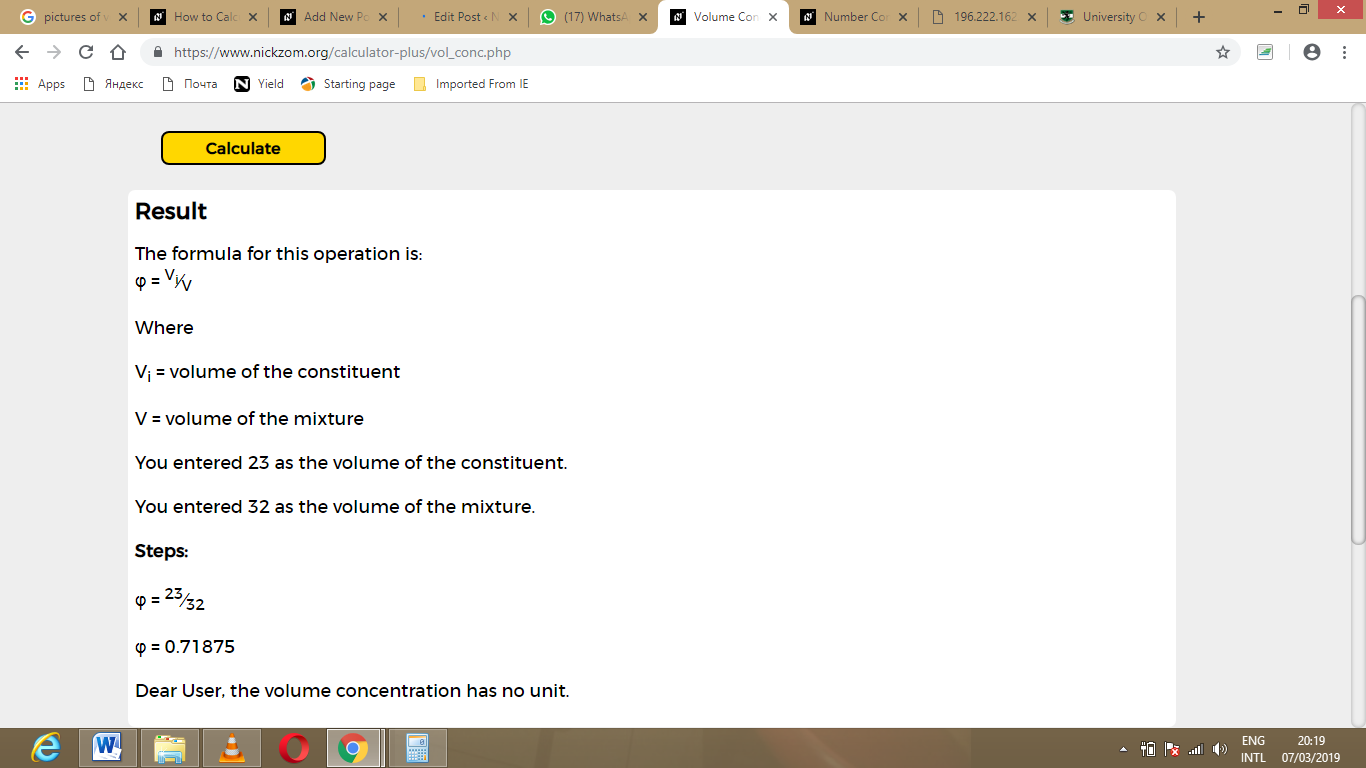
Because neither the indicator word "in" nor a solubility limit is present in the given statement, the relative amounts of helium, He, and molecular chlorine, Cl 2, must be compared to determine which chemical is the solvent and which substance is the solute in this solution. Answer In order to calculate the volume percent of a solution, each substance that is referenced in the problem must first be classified as a solute or a solvent. \)Ĭalculate the volume percent of a solution that is prepared by mixing 519.2 milliliters of helium and 168.4 milliliters of molecular chlorine.


 0 kommentar(er)
0 kommentar(er)
